Today's new automotive battery technologies: Do you understand them?
Content brought to you by Motor Age. To subscribe click here.
What you will learn:
•The differences in the newer battery technologies
•Charging strategies differ between battery types
•Batteries are made for particular applications
The original lead-acid battery has been around as long as the automobile has existed. In fact, some early vehicles were propelled only by this original lead-acid technology and replaced by petroleum after the turn of the century. Of course, there have been some improvements since those early in the beginning. Until recent history, automotive battery design and technology have remained the same because there was little need for improvement.
Times, they are a-changin'
The cars and trucks developed and sold over the last 20 years have placed an increasingly growing demand on the vehicle's electrical system. Just think of the existing components that weren't even imagined in the late '60s. Technology such as autonomous systems, high-powered audio, robust networks, restrictive emission systems, and seat warmers have all placed an increasingly more significant burden on those lead-acid plates. As electrical system demands grew, so did the capacity of the batteries they came equipped with, specifically the AGM, or absorbed glass mat battery.
What is an AGM battery?
AGM batteries were developed in their infancy for electronic and computer backup power, the military, and the air travel industry. Soon, the automotive sector noticed the advantages of AGMs for their reliability, safety, and increased capacity.
AGM batteries can perform the same duties that the old-school flooded batteries excel at, mainly making a strong burst of power to the vehicle's ICE (internal combustion engine) starter but can also support those new electricity demands brought on by the systems described above with their robust bank of long-term, consistent power (Figure 1).
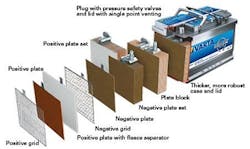
There are some common misconceptions about AGM batteries compared to traditional flooded lead-acid units that they are replacing. Firstly, some still consider flooded lead-acid batteries a better choice than AGM systems. However, AGM batteries are lead acid-based and share most of their capabilities with their flooded cousin. The primary difference in their physical structure is how the electrolyte has direct contact with the battery lead plates. As the name "absorbent glass mat" refers, the AGM battery is constructed with glass cushions between very thin lead plates, allowing the battery to contain many more plates than a traditionally built, lead-acid version. With more lead in the battery, there is a marked increase in the electrical capacity and stability of the battery. The AGM "sponges" that reside between the battery lead plates absorb the battery acid for a more stable and efficient contact between the cells and the electrolyte. AGMs are also sealed, making them maintenance-free and spill-proof.
How AGM batteries work
The fiberglass mat in an AGM battery is sandwiched between the lead plates, wicking the electrolyte between the plates (Figure 2). The mats are designed to expose a controlled amount of acid to the lead plates rather than a saturated, flooded arena. This process allows for closer proximity of the lead plates and a corresponding increase in power storage. These mats also perform an additional feature in reducing the opportunity for an electrolyte spill from a ruptured battery case. There is also a sub-sect of AGM batteries with the plates and electrolyte mat laid out in a spiral format, giving the battery an even greater deep cycle capability.
Advantages of AGM batteries
AGM battery use has been steadily increasing since the '90s has been installed in vehicles since the 1990s, growing in their application at around 8 million batteries per year, and are especially valuable to electric and hybrid electric vehicles due to their inherent support of robust vehicle electrical systems. In addition, due to their sealed nature, they can safely be installed into non-traditional vehicle areas such as in the trunk or under the vehicle seats or floorboards (Figure 3).
Other than the internal structure, two significant differences between the AGM and lead-acid batteries make the AGM design a clear winner. The first is a small valve in the AGM battery case that allows the release of water vapor from the battery. The chemical process inside both AGM and lead-acid batteries can create water vapor which can increase the acidic level of the battery electrolyte if allowed to escape the battery case. AGMs have a valve that prevents those water gases from escaping the battery, keeping Internal balanced chemistry that protects the battery plates and extends the battery's life (Figure 4). This valve also serves as an essential safety feature: preventing over pressurization due to battery overcharging. For example, if an incorrect battery charging schema is used on an AGM battery, it can cause an excess of gas build-up inside the casing. In this scenario, the valve releases the excess internal pressure and prevents a possible case rupture or explosion.
AGMs' second significant advantage is rooted in their basic construction, specifically their fiberglass mats. Traditional lead-acid battery design allows battery electrolytes to free flow through the cells as they interact with the lead plates. AGM fiberglass mats act like sponges, absorbing the electrolyte and directly contacting the lead plates making the battery safer by restricting the electrolyte in the matt instead of a liquid.
Other general advantages include:
Safer transportation, installation, and handling
Greater electrical capacity, resulting in more vehicle "starts"
Faster charging capabilities
More durable
Deep cycle capability
Extreme temperature compatibility
Pressure release valves
More resistant to standard vehicle vibration
Up to three times the lifespan over a lead-acid battery
Sealed, preventing an accidental spillage of battery acids
Maintenance-free
AGM disadvantages
There is only one significant disadvantage of an AGM: the cost. AGMs tend to be significantly more expensive when compared to a lead-acid battery of comparable capacity. In addition, there are a few other minor concerns such as:
AGMs tend to be heavier
AGMs have specific charging requirements that, if ignored, can damage the battery and, in extreme cases, create a catastrophic rupture of the AGM battery case
There are also some safety concerns with AGMs. Because they are produced as a sealed battery, they require specific charging schemas and, if ignored, can cause the AGM to overcharge and explode. This can be easily avoided by assuming that all batteries are AGM, preventing overcharging from taking place.
AGM best practices
From a safety standpoint, there are some best practices a technician can follow to assure that their interaction with all batteries is a successful one.
Assume that every battery you service/replace is an AGM
Use smart chargers whenever servicing an AGM battery low on a charge
Never downgrade the vehicle to a flooded battery if the OEM equipped it with an AGM
Always wear the appropriate personal protective equipment (PPE) when working on or around batteries.
An excellent resource for AGM battery safety can be found with CCAR (Coordinating Committee for Automotive Repair) (Figure 5). CCAR is a not-for-profit organization that works within the automotive industry to provide best practice information for automotive repair shops, the insurance industry, technical colleges, and OEMs. Charlie Ayers and the staff at CCAR offer award-winning, affordable safety and hazardous training on AGM batteries that can make a difference for you and your team. More information on CCAR can be found on their website www.ccar-greenlink.org.
Personal protective equipment and AGM batteries
When servicing AGMs, the personal protective equipment (PPE) you should use does not vary from the requirements for flooded lead-acid units. Acid-resistant gloves, safety glasses, and adequate clothing to protect from an acid spill are always good practices to follow during battery R&R procedures. Yes, we've all ignored correct PPE on occasion, from not wearing eye protection to the proper placement of jack stands and wheel chocks. However, using correct protective equipment for all automotive repair procedures can protect us from minor and major incidents, many of which can be career or even life-ending.
AGM, lithium batteries, and electric vehicles
One of the fastest-growing platforms for AGM batteries and their increased capacity is today's "start-stop" vehicles, including electric and hybrid-electric vehicles. These vehicle configurations require a more effective 12V system to keep the car or truck's auxiliary systems such as air conditioning and ADAS systems operational when the vehicle is at a standstill and the start-stop feature active. As mentioned earlier, the growth of AGM is directly related to new vehicle power requirements from newly developed systems and start-stop and EV systems most definitely fall into that category. It is estimated that over 20 million vehicles on our roads today require an AGM battery for proper operation, and that number continues to climb exponentially. In addition, as newly released models continue to progress with full and mild hybrid systems, always-on technology, and start-stop systems, the demand for AGM power levels will continue to increase. There is, however, a distinct difference between AGM batteries, a recent type of battery technology, and the newest, lithium.
Lithium batteries
Lithium battery technology has become a staple in our daily lives, within and external to the automotive industry. They appear in our laptop computers, cell phones, cordless power tools, and as the power source for today's hybrid and electric vehicles. Like their applications, many different technologies are umbrellaed under the name "Lithium." Still, the technology most adopted by electric vehicles as a propulsion source is lithium-ion.
Lithium-ion batteries are constructed of an anode, a cathode, separator, electrolyte, and the positive and negative terminals, as seen on SLA and AGM batteries. The lithium in the battery is stored in the anode and cathode. The electrolyte carries the positively charged lithium ions from the anode to the cathode and back through the separator. The movement of the ions creates free electrons in the anode at the positive terminal.
Differences between lithium and AGM batteries
The comparison of the two battery technologies addressed in this article is as distinct as the tasks they are assigned to do in the transportation industry. While lithium battery use is expanding into new applications, its primary use is propulsion in a dedicated electric, full hybrid electric, or mild hybrid electric vehicle for cars and trucks.
- Difference #1: The cost. Lead-acid AGM batteries have a significant advantage over lithium batteries for many reasons. AGM batteries are very well priced, easily replaced, and readily available, while lithium automotive batteries are the exact opposite. A trained technician can only perform lithium battery service, which is relatively expensive to replace, and are costly, with some major manufacturer battery replacements approaching the cost of a new subcompact car. However, suppose you look at the price and the power range it represents, and the power-to-weight ratio. In that case, it is easy to see why lithium is the wise choice for vehicle propulsion, and AGM led acid batteries are the correct choice in most circumstances for sealed lead-acid (SLA) application.
- Difference #2: Energy and range. Compared side-by-side, lithium batteries can show an energy density of three to seven or eight times greater than a lead-acid battery. If you were to apply these two batteries as a propulsion energy source in identical vehicles, the lead-acid batteries would take up to ten times the volume of the lithium battery. Additionally, the lead-acid batteries would weigh more than the lithium in a similar ratio. Although early electric vehicles used lead-acid technology for propulsion, they were quickly phased out as batteries with higher energy to weight ratios were developed.
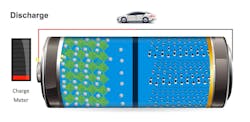
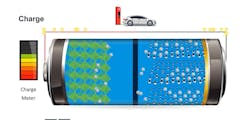
- Difference #3: Charging. If electric vehicles are ever a consumer-friendly direct replacement of gasoline or diesel power, then the time to charge an EV powered by a lithium battery will need to approach the length of time it takes to fill up a gasoline tank (Figures 6+7). Lithium charging appliances are approaching that level of charging speed but have some development to go. However, even at its still-lacking length of charging capability, lithium still has a significant charging advantage over lead-acid and AGM. This time difference is a moving target when attempting to compare. Still, a good rule of thumb today would be that a similarly sized lithium battery can be charged at least four times faster than a comparable lead-acid battery bank (Figure 8).